- Science & Math
- Sociology & Philosophy
- Law & Politics

Lab Answers: Energy from Burning Food
- Lab Answers: Energy from Burning…
If the change in temperature is greater when the water is heated with the use of the fire caught by the food substance, then the energy content in the food substance is higher because the heat energy is greater, since the heat energy is absorbed by the water when the fire is kept under the test tube containing water.
The formula indicates that if the change in temperature is greater when the mass of the substances and the volume of water are constant, then the heat energy is higher.
The conclusion drawn by my hypothesis is:
- Measuring Cylinder
- Laboratory Thermometer
- Needle with Handle
- Scalpel (for cutting the substances into exactly 0.5 grams)
- Test tube holder
- The following substances are the 5 different food items that are used to conduct the experiment, the substances used are:
i. Biscuit
ii. Koko Crunch
iii. Cheetos
iv. Peanut
v. Candle nut
- Independent Variable: Heat energy of the food substance used.
- Dependent Variable: Temperature change in the water/Amount of energy absorbed.
- Controlled Variable: Amount of water, Temperature of surroundings, Type of needle used, Temperature of water.
Manipulation
- Independent Variable: As we vary the food items that we use, their heat energy/ they themselves become the independent variable.
- Dependent Variable: The change in temperature/ Heat energy absorbed is varied as the heat energy of the substance is varied.
- Controlled Variable: The temperature is not varied in any case or does not depend on anything during this experiment, the amount of water equals 20ml in each trial of an experiment for each food substance.
- Measure 20ml water in the measuring cylinder and pour it into the test tube.
- Place the test tube in the holder and lock it tight.
- If the food substance measures 0.5 grams on the electrical balance, then use the substance, otherwise use the scalpel to divide it into smaller pieces and make sure it measures exactly 0.5 grams.
- Measure the initial temperature of the water using the thermometer
- Poke through a food substance measuring 0.5 grams using the needle with the handle.
- Turn on fire on the burner.
- Set the food substance on the needle to fire on the burner.
- Once the food substance starts to burn, place it under the test tube so the water inside it can absorb heat.
- Measure the temperature change in the water using the thermometer.
- Measure the energy content in the food item by using the following formula:
Average Results
Discussion of results.
The least energy as the graph shows is in the Cereal (Koko Crunch). It contains about 1.2 kJ of Average Energy. Candlenut contains the highest amount of energy in the 5 items used during the experiment possessing energy of approximately 8.6kJ.
The trials of the Biscuit show increasing energy from T1 to T3, causing the Average Energy to be higher than the energy obtained in T1 and T2 but lesser than T3. The results of Candlenut show a similar pattern and Peanuts have an opposite pattern.
The results of Cheetos show a pattern of results being T1 (Least) – T2 (Highest) – T3 (Lesser than Highest and Higher than Least). The Average Energy in this case is just a bit higher than the T3. The Koko Crunch shows the opposite pattern and therefore the Average Energy observed is higher than T3.
The trials of Biscuit and Peanut show high variation, this shows the inaccuracy in the results that can be explained by evaluating the method used.
My results completely agree with my hypothesis that when the temperature change is greater, the energy content is higher. My hypothesis states:
If compared to my results, I can vouchsafe that my hypothesis agrees with my results.
The experiment was done with the best method possible in the lab with the provided equipment. The accuracy could be increased by:
- Use a calorimeter to insulate the test tube to prevent loss of heat energy.
- Use a digital thermometer for accurate readings of temperature.
- Prevent the carbon coating that is formed on the test tube when a substance is burnt as it forms insulation.
- Try to have a handle made out of wood for the needle as metal conducts heat.
- Conduct more trials.
- Turn off the A/C and perform the experiment at room temperature.
- Use exactly 0.5 grams of food substances. Prevent even the minute errors.
Related Posts
- Determining Heat Capacity of Water Lab Answers
- Copper Penny to Silver Lab Answers
- Phet Projectile Motion Lab: Lab Answers
- Physics: Conservation of Energy Lab Answers
- Energy Content of Food Lab Report Answers
12 Comments
Lovely, just lovely. A true champion in the field of science.
what is the name of this experiment?
Extremely useful!!!
AMAZING EXPERIMENT! schoolwork helper, thank you for helping me understand calories
what is left at the end of the experiment when the foods are completely burnt?
you used 5.839 as your average for the peanut, it should be 5.389. 🙂
Calculation of energy content of peanut
What formula do you use to calculate the amount of Joules?
Energy released from food ( gram) = (Mass of water x temp rise x 4.2g) / Mass of food sample
Really great .. thank you so much
Thanks a lot this helped me with my plan and design lab.Thanks much really appreciate it 😊😊
Thanks a lot!! I really appreciate it!!!! It helped me with my homework and to be frank, it fulfilled my requirements!! Please keep it up!! 😉
Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.
Post comment
Your browser is not supported
Sorry but it looks as if your browser is out of date. To get the best experience using our site we recommend that you upgrade or switch browsers.
Find a solution
- Skip to main content
- Skip to navigation

- Back to parent navigation item
- Primary teacher
- Secondary/FE teacher
- Early career or student teacher
- Higher education
- Curriculum support
- Literacy in science teaching
- Periodic table
- Interactive periodic table
- Climate change and sustainability
- Resources shop
- Collections
- Remote teaching support
- Starters for ten
- Screen experiments
- Assessment for learning
- Microscale chemistry
- Faces of chemistry
- Classic chemistry experiments
- Nuffield practical collection
- Anecdotes for chemistry teachers
- On this day in chemistry
- Global experiments
- PhET interactive simulations
- Chemistry vignettes
- Context and problem based learning
- Journal of the month
- Chemistry and art
- Art analysis
- Pigments and colours
- Ancient art: today's technology
- Psychology and art theory
- Art and archaeology
- Artists as chemists
- The physics of restoration and conservation
- Ancient Egyptian art
- Ancient Greek art
- Ancient Roman art
- Classic chemistry demonstrations
- In search of solutions
- In search of more solutions
- Creative problem-solving in chemistry
- Solar spark
- Chemistry for non-specialists
- Health and safety in higher education
- Analytical chemistry introductions
- Exhibition chemistry
- Introductory maths for higher education
- Commercial skills for chemists
- Kitchen chemistry
- Journals how to guides
- Chemistry in health
- Chemistry in sport
- Chemistry in your cupboard
- Chocolate chemistry
- Adnoddau addysgu cemeg Cymraeg
- The chemistry of fireworks
- Festive chemistry
- Education in Chemistry
- Teach Chemistry
- On-demand online
- Live online
- Selected PD articles
- PD for primary teachers
- PD for secondary teachers
- What we offer
- Chartered Science Teacher (CSciTeach)
- Teacher mentoring
- UK Chemistry Olympiad
- Who can enter?
- How does it work?
- Resources and past papers
- Top of the Bench
- Schools' Analyst
- Regional support
- Education coordinators
- RSC Yusuf Hamied Inspirational Science Programme
- Science Education Policy Alliance
- RSC Education News
- Supporting teacher training
- Interest groups

- More navigation items
Energy content in foods
In association with Nuffield Foundation
- Four out of five
- No comments
Try this class experiment to investigate how much energy different foods contain
In this practical, students burn a sample of a foodstuff of known mass, heating a known volume of water. From the measured temperature change, students calculate the energy transferred to the water, and hence estimate the energy present per unit mass of food.
This is a class experiment in which different groups can investigate different foodstuffs. If each group investigates two foodstuffs – one in common with the rest of class to provide a common baseline, and the other a different foodstuff from the rest – a comparative table of energy in different foods can be drawn up from the class results. This should be possible to achieve in 45–60 minutes.
- Eye protection
- Thermometer (–10 to 110 °C), short, stirring type
- Boiling tube, or metal calorimeter (or similar metal container) (see note 4 below)
- Measuring cylinder, 25 cm 3
- Bunsen burner
- Heat resistant mat
- Mounted needle
- Stand and clamp
- Balance, weighing to 0.1 g
Use of a variety of dry foodstuffs, such as:
- Mini-marshmallows
- Popcorn (already popped)
- Broad beans (dried)
See notes 5, 6 and 7 below for additional guidance.
Health, safety and technical notes
- Read our standard health and safety guidance.
- Wear eye protection throughout.
- Students must be instructed NOT to taste or eat any of the foods used in the experiment.
- While boiling tubes are easy to use for this experiment, the poor thermal conductivity of glass may be a major cause of error. Metal containers, such as copper calorimeters or tin cans of similar dimensions that can be held in a clamp, provide more effective heat transfer to the water. Note that this benefit is lost if the can is stood on a tripod, as the latter will also be heated.
- Check in advance for common allergy problems, eg peanuts.
- Some foodstuffs can be burned safely and easily using a mounted needle. Others may melt and drop off the needle, so burning on an old metal teaspoon is an alternative method – this can also be used for liquid foodstuffs, such as olive oil. High protein foodstuffs may produce pungent fumes, and should be burned in a fume cupboard. Each foodstuff provided should be tested beforehand to check that it is capable of sustained combustion without having to be relit repeatedly.
- One foodstuff needs to be selected as suitable for the standard experiment used by each group. Enough samples of approximately the same mass of this foodstuff need to be provided for the class. A trial run before the lesson should be carried out to establish that it will burn readily, sustain combustion and leave little unburnt residue, and that the mass of this foodstuff that will cause a temperature rise in the water used of around 20–30 °C.
Using a test tube
- Measure 10 cm 3 of water into the test tube.
- Clamp the test tube in the retort stand at an angle as shown in the diagram, and over a heat resistant mat.
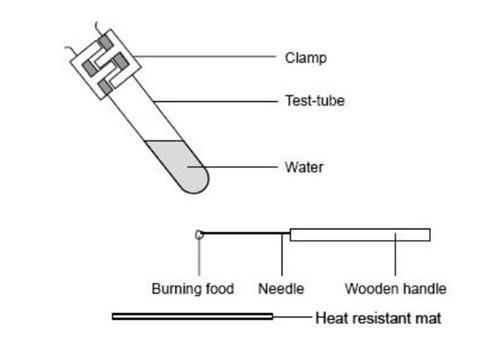
Source: Royal Society of Chemistry
The equipment required to investigate the energy content of different foodstuffs
- Weigh a small piece of food and record the mass.
- Take the temperature of the water and record it in the table.
- Fix the food on the end of the mounted needle. If the food is likely to melt when heated put it on a teaspoon instead of on the needle.
- Ignite the food using a Bunsen burner, and immediately hold it about 1 cm below the test tube and above a heat resistant mat. If the flame goes out, quickly relight it.
- When the food stops burning, stir the water with the thermometer and record the temperature.
- If there is a significant amount of unburnt food left on the needle, reweigh this and record the mass remaining.
- Empty the test tube and refill it with another 10 cm 3 of cold water. Repeat the experiment using a different food.
Using a metal container
Instructions as for a test tube, except:
- Use a larger volume of water, eg 25 or 50 cm 3 , and a larger food sample.
- Clamp the container in a level position above a heat resistant mat.
Teaching notes
This experiment provides an opportunity to use a temperature sensor linked to a data logger instead of a thermometer.
One foodstuff should be preselected to be the one used by all groups to standardise their experiments with each other (see Health, safety and technical notes ), while each group needs to be allocated a different foodstuff for the second run.
As the same amount of water is heated each time, the temperature rise can be used to compare the amount of heat energy given off per gram of each foodstuff by dividing the rise by the mass of foodstuff burnt.
For classes familar with the equation q = m × C × Δ T , where q is the heat energy, m the mass of water heated, C the specific heat of water (4.2 J g –1 deg – 1 ) and Δ T the temperature rise, this can be used to calculate the energy (in J) absorbed by the water each time. Dividing this by the mass of foodstuff burnt gives the heat energy absorbed by the water in J per g, as shown in the table below.
Each group needs to prepare a results table along the lines of the example below:
Class results for the heat absorbed by water per gram of food may then be collected and compared on a class spreadsheet prepared by the teacher. After this has been done, a class discussion of ‘fair test’ problems will be appropriate, identifying sources of error and ideas for improving the technique used.
More resources
Add context and inspire your learners with our short career videos showing how chemistry is making a difference .
Additional information
This is a resource from the Practical Chemistry project , developed by the Nuffield Foundation and the Royal Society of Chemistry. This collection of over 200 practical activities demonstrates a wide range of chemical concepts and processes. Each activity contains comprehensive information for teachers and technicians, including full technical notes and step-by-step procedures. Practical Chemistry activities accompany Practical Physics and Practical Biology .
© Nuffield Foundation and the Royal Society of Chemistry
- 11-14 years
- 14-16 years
- 16-18 years
- Practical experiments
- Thermodynamics
- Quantitative chemistry and stoichiometry
- Analytical chemistry
Specification
- The quantity of heat energy released can be determined experimentally and calculated using Eₕ=cmΔT.
- Fats and oils are: a concentrated source of energy; essential for the transport and storage of fat-soluble vitamins in the body.
- 2.8.7 calculate enthalpy changes from experimental data using the equation q = mcΔT;
- determine the enthalpy changes for combustion and neutralisation using simple apparatus; and
- 2. Develop and use models to describe the nature of matter; demonstrate how they provide a simple way to to account for the conservation of mass, changes of state, physical change, chemical change, mixtures, and their separation.
- 9. Consider chemical reactions in terms of energy, using the terms exothermic, endothermic and activation energy, and use simple energy profile diagrams to illustrate energy changes.
Related articles

Make worked examples count in quantitative chemistry
2024-12-11T06:36:00Z By Helen Skelton
Four teacher-tested approaches to encourage self-explanation and build learners’ confidence, engagement and understanding

Teaching conservation of mass at 14–16
2024-12-05T07:33:00Z By Matthew Parks
Help learners master the concept of conservation of mass with these hands-on teaching ideas

Scientists find hazardous pigments in tattoo ink
2024-11-29T08:30:00Z By Nina Notman
Use this science research context when studying instrumental methods with your 14–16 learners
No comments yet
Only registered users can comment on this article., more experiments.


‘Gold’ coins on a microscale | 14–16 years
By Dorothy Warren and Sandrine Bouchelkia
Practical experiment where learners produce ‘gold’ coins by electroplating a copper coin with zinc, includes follow-up worksheet

Practical potions microscale | 11–14 years
By Kirsty Patterson Four out of five
Observe chemical changes in this microscale experiment with a spooky twist.

Antibacterial properties of the halogens | 14–18 years
By Kristy Turner
Use this practical to investigate how solutions of the halogens inhibit the growth of bacteria and which is most effective
- Contributors
- Email alerts
Site powered by Webvision Cloud
- High School
- You don't have any recent items yet.
- You don't have any courses yet.
- You don't have any books yet.
- You don't have any Studylists yet.
- Information
Lab 18 (DRY) - This is a lab report, which gives the procedure, data and conclusion.
General chemistry laboratory (chem 106), hunter college cuny.
Recommended for you
Students also viewed.
- Lab 19 (WET) - This is a lab report, which gives the procedure, data and conclusion.
- - Post Lab 1 - Post Lab 1 for Chemistry 106. Worth 30 points.
- CHEM 106 lab 11 and 12
- Lab 11+12 - Grade: 95
- Syllabus 106docx - Lab
- Chem - lab 1 - lab 1
Related documents
- Chem106 Lab #9 
 - General Chemistry Lab #9 and #10
- Chem106 Lab 8 - General Chemistry Lab #8
- Chem106 Lab#5-6 - General Chemistry Lab #5 and #6
- Sam Jones Lab Report 6
- Lab 15 Quiz - Lab quiz
- Lab 5 How can we build molecules? “Models to the rescue”
Preview text
Neha Jacob TA Edmund Date: 05-09- Experiment 18: “Burning Food. Where are my carbs?” Introduction: The process of consuming food and digesting it allows for our body to convert the food into energy, through chemical reactions, which releases energy to fuel our bodies. This works through the use of oxygen for a combustion reaction. In this lab, we will use data from bomb calorimetry experiments which allow us to determine the heat released through the process of burning food, and this is done by using volume-constant calculations. We initially determined the calorimeter constant using glucose, and then we saw how much energy was released when food burned in our bodies, and we found the caloric contents of various snacks that we consume. We will also identify patterns in the calorie or energy content of snack foods. This experiment will allow us to understand the process of burning food and how it is converted to energy. This lab can also expand our attention to the calories we intake on a daily basis so that we can take in the healthier calories for a healthier body. Materials:
- Laboratory manual
- Calculator Observations: Part 1:Calculations: C 6 H 12 O 6 + 6O 2 → 6CO 2 + 6H 2 O ΔHf (glucose) kJ/mol ΔHf (CO 2 ) kJ/mol ΔHf (H 2 O(g)) kJ/mol ΔHrxn kJ/mol -1275 -393 -242 - ΔHrxn = (6 x-242) + (6 x -393) - (-1275) = -2538 kJ/mol Calculations: n(glu) = 3 x 1 𝑚𝑜𝑙𝑒/180 = 0 mol glucose
ΔT = 29 - 24 = 4
Qcombustion = 0 mol - 2538 kJ/mol = -42 kJ Qcalorimeter = 42 kJ Ccal = 42.26kJ/4 = 9 kJ/C x 1000J = 9828 J/C = 9 kJ/C sample m(glu) g n(glu) Initial T Final T q(comb) kJ q(cal) kJ ΔT oC C(cal) kJ/oC glucose 3 0. 7
24 29 -42 42 4 9.
Part 2: Food Sample Mass (g) Initial T (oC) Final T (oC)
oC q(cal) kJ q(comb) kJ Energy Content kJ/g Energy Content Cal/oz Label Cal/oz
Error Oreo cookies 2 26 30 4 47 -47 19 132 133 0. Microwave popcorn
2 25 29 4 41 -41 17 121 122 0.
Crunchy Cheetos
2 25 30 5 52 -52 23 161 160 1.
Beef jerky 2 25 29 3 45 -35 16 109 80 36.
beef jerky, and oil-roasted, salty peanuts. The results came out to be 132 Cal/oz, 121. Cal/oz, 161 Cal/oz, 109 Cal/oz, and 167 Cal/oz. We concluded that beef jerky had the lowest energy content while the oil-roasted, salted peanuts had the highest energy content. This makes sense because the peanuts were roasted in oil and were salted which concludes that there must be more calories. This lab can be used in real life when we are making decisions on what to eat or snack throughout the day. We should be observant of calories if we want to live a healthier life. Focus Questions: 1. The amount of energy released from food, is the amount that each food varies depending what types of macromolecules it is composed from. Macromolecules such as fats, carbohydrates, or proteins. When food is burned, all the energy available is released. 2. The caloric content is determined by energy released from food when it’s burned. We can determine those values by the combustion reaction using a calorimeter. Post Lab Questions: 1. The oil roasted, salted peanuts had the highest number of calories per gram. I was not surprised by this finding because they are high in fat and oily. 2. Oil roasted salted peanuts have the most stored energy. 3. The reason why we should calculate calories per gram rather than calories burned is that we can make direct comparisons to other samples. “Calories burned” is a very vague measurement and can easily be misleading. 4. 5 / 342 (g/mol) = 0 (27 - 23) x 4 = 20 -20 / 0 = -2030 kj/mol References: Smeureanu, G. & Geggier, S. (2022) General Chemistry Laboratory. New York, NY.
- Multiple Choice
Course : General Chemistry Laboratory (CHEM 106)
University : hunter college cuny.

- More from: General Chemistry Laboratory CHEM 106 Hunter College CUNY 999+ Documents Go to course
- CCEA Double Award
B2: Food energy Practical B2 - Investigate energy content of food
How to investigate the energy content of food by burning food samples.
Part of Combined Science Prescribed practicals
Save to My Bitesize
Practical B2 - Investigate the energy content of food
A simple investigation can be conducted to investigate the energy content of a food sample.
- Add water - around 20cm 3 - to a boiling tube clamped in a retort stand.
- Record the starting temperature of the water.
- Place food sample on mounted needle.
- Ignite the food sample using a Bunsen burner.
- Hold the burning food sample under the boiling tube of water until completely burned – it may be necessary to relight the food sample.
- Record the final temperature of the water.
- Record results in a table.
- Calculate the change in temperature caused by the burning food sample.
- Repeat steps 1- 8 with this food type to increase reliability.
- Calculate the average change in temperature for this food type.
- Calculate the energy released by this food type using this equation: Energy released (J) = mass of water (g) x rise in temperature (°C) x 4.2
- Repeat steps 1-8 with different food types for comparison.
More guides on this topic
- B1: Photosynthesis
- B3: Enzymes
- B4: Habitats
- B5: Osmosis
- B6: Measuring water uptake and water loss
Related links
- Combined Science Exam practice
- Personalise your Bitesize!
- Jobs that use Science
- BBC: Science and Environment
- Save My Exams Subscription
- Tassomai Subscription
- Headsqueeze
- Revision Buddies Subscription
- International
- Education Jobs
- Schools directory
- Resources Education Jobs Schools directory News Search
Energy in food (burning food practical) KS3 or KS4
Subject: Biology
Age range: 11-14
Resource type: Other
Last updated
27 January 2017
- Share through email
- Share through twitter
- Share through linkedin
- Share through facebook
- Share through pinterest

Creative Commons "Sharealike"
Your rating is required to reflect your happiness.
It's good to leave some feedback.
Something went wrong, please try again later.
jeraldinefrost
Brilliant resource. Many thanks.
Empty reply does not make any sense for the end user
simple , easy and clear for all levels. I forgot engaging
trevorgoode0
Top man, Piotr!!
lennie_visbal1965
Thanks so much!
Report this resource to let us know if it violates our terms and conditions. Our customer service team will review your report and will be in touch.
Not quite what you were looking for? Search by keyword to find the right resource:

IMAGES
COMMENTS
Apr 1, 2017 · Poke through a food substance measuring 0.5 grams using the needle with the handle. Turn on fire on the burner. Set the food substance on the needle to fire on the burner. Once the food substance starts to burn, place it under the test tube so the water inside it can absorb heat. Measure the temperature change in the water using the thermometer.
When the food stops burning, stir the water with the thermometer and record the temperature. If there is a significant amount of unburnt food left on the needle, reweigh this and record the mass remaining. Empty the test tube and refill it with another 10 cm 3 of cold water. Repeat the experiment using a different food. Using a metal container
Neha Jacob TA Edmund Date: 05-09- Experiment 18: “Burning Food. Where are my carbs?” Introduction: The process of consuming food and digesting it allows for our body to convert the food into energy, through chemical reactions, which releases energy to fuel our bodies. This works through the use of oxygen for a combustion reaction.
Calculate the change in temperature caused by the burning food sample. Repeat steps 1- 8 with this food type to increase reliability. Calculate the average change in temperature for this food type.
the burning food item. This method indirectly measures the amount of heat given off by the food. Purpose: To calculate how many calories of energy/ serving food items contain & compare to real number as determined by the manufacturer. Determine how this experiment can be improved to provide more accurate results. Materials:
Apr 9, 2024 · We will repeat the investigation several times for each food sample; Measurement 1 We will measure the change in temperature of the water; Measurement 2 The mass of the food will be measured after the food sample has burned out; Same. We will control the volume of water used and the distance between the food sample and the boiling tube during ...
and the food. 5 Get the food to burn in a Bunsen flame and immediately put it back under the test tube. 6 When the food stops burning, stir the water gently ith the themete, and mere the inal tepeatre. 7 If you have time, repeat the experiment with other foods, e.g. nuts, bread, rice, spaghetti. Note that some
Different foods have different energy contents. The energy content of a food can be released when you set it alight. When you hold a burning food underneath a known volume of water, the temperature increase can be measured. A simple calculation can then be used to estimate the amount of energy stored within the food. Apparatus 25 cm. 3 ...
Hold the burning food sample under the boiling tube of water until completely burned – it may be necessary to relight the food sample. Record the final temperature of the water. Record results ...
Jan 27, 2017 · A simple version of 'burning food' practical with the instructions, table of results, scaffold for the conclusions and suggested points of improvement. This can be used with KS3 or low level KS4 groups of students.