John Dalton
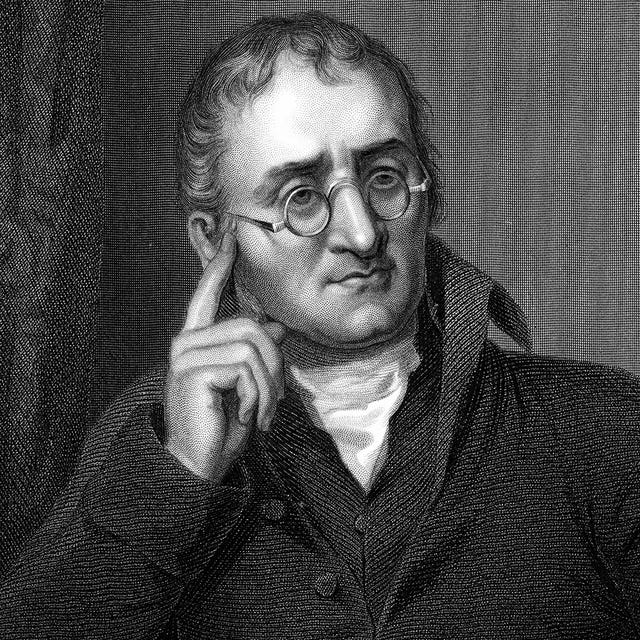
(1766-1844)

Who Was John Dalton?
During John Dalton's early career, he identified the hereditary nature of red-green color blindness. In 1803 he revealed the concept of Dalton’s Law of Partial Pressures. Also in the 1800s, he was the first scientist to explain the behavior of atoms in terms of the measurement of weight.
Early Life and Career
Dalton was born in Eaglesfield, England, on September 6, 1766, to a Quaker family. He had two surviving siblings. Both he and his brother were born color-blind. Dalton's father earned a modest income as a handloom weaver. As a child, Dalton longed for formal education, but his family was very poor. It was clear that he would need to help out with the family finances from a young age.
After attending a Quaker school in his village in Cumberland, when Dalton was just 12 years old he started teaching there. When he was 14, he spent a year working as a farmhand but decided to return to teaching — this time as an assistant at a Quaker boarding school in Kendal. Within four years, the shy young man was made principal of the school. He remained there until 1793, at which time he became a math and philosophy tutor at the New College in Manchester.
While at New College, Dalton joined the Manchester Literary and Philosophical Society. Membership granted Dalton access to laboratory facilities. For one of his first research projects, Dalton pursued his avid interest in meteorology. He started keeping daily logs of the weather, paying special attention to details such as wind velocity and barometric pressure—a habit Dalton would continue all of his life. His research findings on atmospheric pressure were published in his first book, Meteorological Findings , the year he arrived in Manchester.
During his early career as a scientist, Dalton also researched color blindness—a topic with which he was familiar through firsthand experience. Since the condition had affected both him and his brother since birth, Dalton theorized that it must be hereditary. He proved his theory to be true when genetic analysis of his own eye tissue revealed that he was missing the photoreceptor for perceiving the color green. As a result of his contributions to the understanding of red-green color blindness, the condition is still often referred to as "Daltonism."
Dalton's Law
Dalton's interest in atmospheric pressures eventually led him to a closer examination of gases. While studying the nature and chemical makeup of air in the early 1800s, Dalton learned that it was not a chemical solvent, as other scientists had believed. Instead, it was a mechanical system composed of small individual particles that used pressure applied by each gas independently.
Dalton's experiments on gases led to his discovery that the total pressure of a mixture of gases amounted to the sum of the partial pressures that each individual gas exerted while occupying the same space. In 1803 this scientific principle officially came to be known as Dalton's Law of Partial Pressures. Dalton's Law primarily applies to ideal gases rather than real gases, due to the elasticity and low particle volume of molecules in ideal gases. Chemist Humphry Davy was skeptical about Dalton's Law until Dalton explained that the repelling forces previously believed to create pressure only acted between atoms of the same sort and that the atoms within a mixture varied in weight and complexity.
The principle of Dalton's Law can be demonstrated using a simple experiment involving a glass bottle and large bowl of water. When the bottle is submerged under water, the water it contains is displaced, but the bottle isn't empty; it's filled with the invisible gas hydrogen instead. The amount of pressure exerted by the hydrogen can be identified using a chart that lists the pressure of water vapors at different temperatures, also thanks to Dalton's discoveries. This knowledge has many useful practical applications today. For instance, scuba divers use Dalton's principles to gauge how pressure levels at different depths of the ocean will affect the air and nitrogen in their tanks.
During the early 1800s, Dalton also postulated a law of thermal expansion that illustrated the heating and cooling reaction of gases to expansion and compression. He garnered international fame for his additional study using a crudely fashioned dew point hygrometer to determine how temperature impacts the level of atmospheric water vapor.
Atomic Theory
Dalton's fascination with gases gradually led him to formally assert that every form of matter (whether solid, liquid or gas) was also made up of small individual particles. He referred to the Greek philosopher Democritus of Abdera's more abstract theory of matter, which had centuries ago fallen out of fashion, and borrowed the term "atomos" or "atoms" to label the particles. In an article he wrote for the Manchester Literary and Philosophical Society in 1803, Dalton created the first chart of atomic weights.
Seeking to expand on his theory, he readdressed the subject of atomic weight in his book A New System of Chemical Philosophy , published in 1808. In A New System of Chemical Philosophy , Dalton introduced his belief that atoms of different elements could be universally distinguished based on their varying atomic weights. In so doing, he became the first scientist to explain the behavior of atoms in terms of the measurement of weight. He also uncovered the fact that atoms couldn't be created or destroyed.
Dalton's theory additionally examined the compositions of compounds, explaining that the tiny particles (atoms) in a compound were compound atoms. Twenty years later, chemist Amedeo Avogadro would further detail the difference between atoms and compound atoms.
In A New System of Chemical Philosophy , Dalton also wrote about his experiments proving that atoms consistently combine in simple ratios. What that meant was that the molecules of an element are always made up of the same proportions, with the exception of water molecules.
In 1810 Dalton published an appendix to A New System of Chemical Philosophy . In it he elaborated on some of the practical details of his theory: that the atoms within a given element are all exactly the same size and weight, while the atoms of different elements look—and are—different from one other. Dalton eventually composed a table listing the atomic weights of all known elements.
His atomic theories were quickly adopted by the scientific community at large with few objections. "Dalton made atoms scientifically useful," asserted Rajkumari Williamson Jones, a science historian at the University of Manchester Institute of Science and Technology. Nobel Laureate Professor Sir Harry Kroto, noted for co-discovering spherical carbon fullerenes, identified the revolutionary impact of Dalton's discoveries on the field of chemistry: "The crucial step was to write down elements in terms of their atoms...I don't know how they could do chemistry beforehand, it didn't make any sense."
From 1817 to the day he died, Dalton served as president of the Manchester Literary and Philosophical Society, the organization that first granted him access to a laboratory. A practitioner of Quaker modesty, he resisted public recognition; in 1822 he turned down elected membership to the Royal Society. In 1832 he did, however, begrudgingly accept an honorary Doctorate of Science degree from the prestigious Oxford University. Ironically, his graduation gown was red, a color he could not see. Fortunately for him, his color blindness was a convenient excuse for him to override the Quaker rule forbidding its subscribers to wear red.
In 1833 the government granted him a pension, which was doubled in 1836. Dalton was offered another degree, this time a Doctorate of Laws, by Edinburgh University in 1834. As if those honors were insufficient tribute to the revolutionary chemist, in London, a statue was erected in Dalton's honor--also in 1834. "Dalton was very much an icon for Manchester," said Rajkumari Williams Jones. "He is probably the only scientist who got a statue in his lifetime."
In his later life, Dalton continued to teach and lecture at universities throughout the United Kingdom, although it is said that the scientist was an awkward lecturer with a gruff and jarring voice. Throughout his lifetime, Dalton managed to maintain his nearly impeccable reputation as a devout Quaker. He lived a humble, uncomplicated life focusing on his fascination with science, and never married.
In 1837 Dalton had a stroke. He had trouble with his speech for the next year.
Death and Legacy
After suffering a second stroke, Dalton died quietly on the evening of July 26, 1844, at his home in Manchester, England. He was provided a civic funeral and granted full honors. A reported 40,000 people attended the procession, honoring his contributions to science, manufacturing and the nation's commerce.
By finding a way to "weigh atoms," John Dalton's research not only changed the face of chemistry but also initiated its progression into a modern science. The splitting of the atom in the 20th century could most likely not have been accomplished without Dalton laying the foundation of knowledge about the atomic makeup of simple and complex molecules. Dalton's discoveries also allowed for the cost-efficient manufacturing of chemical compounds, since they essentially give manufacturers a recipe for determining the correct chemical proportions in a given compound.
The majority of conclusions that made up Dalton's atomic theory still stand today.
"Now with nanotechnology, atoms are the centerpiece," said Nottingham University Professor of Chemistry David Garner. "Atoms are manipulated directly to make new medicines, semiconductors and plastics." He went on to further explain, "He gave us the first understanding of the nature of materials. Now we can design molecules with a pretty good idea of their properties."
In 2003, on the bicentennial of Dalton's public announcement of his atomic theory, the Manchester Museum held a tribute to the man, his life and his groundbreaking scientific discoveries.
QUICK FACTS
- Name: John Dalton
- Birth Year: 1766
- Birth date: September 6, 1766
- Birth City: Eaglesfield
- Birth Country: United Kingdom
- Gender: Male
- Best Known For: Chemist John Dalton is credited with pioneering modern atomic theory. He was also the first to study color blindness.
- Journalism and Nonfiction
- Science and Medicine
- Education and Academia
- Astrological Sign: Virgo
- John Fletcher's Quaker grammar school
- Death Year: 1844
- Death date: July 26, 1844
- Death City: Manchester
- Death Country: United Kingdom
We strive for accuracy and fairness.If you see something that doesn't look right, contact us !
CITATION INFORMATION
- Article Title: John Dalton Biography
- Author: Biography.com Editors
- Website Name: The Biography.com website
- Url: https://www.biography.com/scientists/john-dalton
- Access Date:
- Publisher: A&E; Television Networks
- Last Updated: May 21, 2021
- Original Published Date: April 2, 2014
- Berzelius' symbols are horrifying. A young student in chemistry might as soon learn Hebrew as make himself acquainted with them.
- We might as well attempt to introduce a new planet into the solar system, or to annihilate one already in existence, as to create or destroy a particle of hydrogen.
- The principal failing in [Sir Humphrey Davy's] character as a philosopher is that he does not smoke.
- I can now enter the lecture room with as little emotion nearly as I can smoke a pipe with you on Sunday or Wednesday evenings.
- Matter, though divisible in an extreme degree, is nevertheless not infinitely divisible. That is, there must be some point beyond which we cannot go in the division of matter... I have chosen the word 'atom' to signify these ultimate particles.
- Will it not be thought remarkable that in 1836 the British chemists are ignorant whether attraction, repulsion or indifference is marked when a mixture of any proportions of azote and oxygen are made.
- In short, [London] is a most surprising place, and worth one's while to see once; but the most disagreeable place on earth for one of a contemplative turn to reside in constantly.
- To ascertain the exact quantity of water in a given quantity of air is, I presume, an object not yet fully attained.
- The cause of rain is now, I consider, no longer an object of doubt.

Famous British People

Ralph Fiennes

Liam Payne’s Girlfriend Speaks Out After His Death

Daniel Day-Lewis

Maggie Smith

Alan Cumming

Olivia Colman

Richard III

20 Shakespeare Quotes

William Shakespeare

- Scientific Biographies
John Dalton
The theory of atomism, proposed by Dalton in the early 19th century and derived from meteorological studies, is the foundation for our modern concept of the atom.

Although a schoolteacher, a meteorologist, and an expert on color blindness, John Dalton is best known for his pioneering theory of atomism. He also developed methods to calculate atomic weights and structures and formulated the law of partial pressures.
Dalton (1766–1844) was born into a modest Quaker family in Cumberland, England, and for most of his life—beginning in his village school at the age of 12—earned his living as a teacher and public lecturer. After teaching for 10 years at a Quaker boarding school in Kendal, he moved on to a teaching position in the burgeoning city of Manchester.
There he joined the Manchester Literary and Philosophical Society, which provided him with a stimulating intellectual environment and laboratory facilities. The first paper he delivered before the society was on color blindness, which afflicted him and is sometimes still called Daltonism.
Theories of Atomism and the Law of Partial Pressures
Dalton arrived at his view of atomism by way of meteorology, in which he was seriously interested for a long period: he kept daily weather records from 1787 until his death, his first book was Meteorological Observations (1793), and he read a series of papers on meteorological topics before the Literary and Philosophical Society between 1799 and 1801.
The papers contained Dalton’s independent statement of Charles’s law (see Joseph Louis Gay-Lussac ): “all elastic fluids expand the same quantity by heat.” He also clarified what he had pointed out in Meteorological Observations —that the air is not a vast chemical solvent as Antoine-Laurent Lavoisier and his followers had thought, but a mechanical system, where the pressure exerted by each gas in a mixture is independent of the pressure exerted by the other gases, and where the total pressure is the sum of the pressures of each gas.

In explaining the law of partial pressures to skeptical chemists of the day—including Humphry Davy —Dalton claimed that the forces of repulsion thought to cause pressure acted only between atoms of the same kind and that the atoms in a mixture were indeed different in weight and “complexity.”
Experiments on Atomic Weights and Structures
He proceeded to calculate atomic weights from percentage compositions of compounds, using an arbitrary system to determine the likely atomic structure of each compound. If there are two elements that can combine, their combinations will occur in a set sequence. The first compound will have one atom of A and one of B; the next, one atom of A and two atoms of B; the next, two atoms of A and one of B; and so on.
Hence, water is HO. Dalton also came to believe that the particles in different gases had different volumes and surrounds of caloric, thus explaining why a mixture of gases—as in the atmosphere—would not simply layer out but was kept in constant motion. Dalton consolidated his theories in his New System of Chemical Philosophy (1808–1827).
As a Quaker, Dalton led a modest existence, although he received many honors later in life. In Manchester more than 40,000 people marched in his funeral procession.
Featured image: Portrait print of Dr. John Dalton, F.R.S. , 1834. Science History Institute
Browse more biographies
Maurice Wilkins

Rachel Fuller Brown

Ellen H. Swallow Richards
Copy the above HTML to republish this content. We have formatted the material to follow our guidelines, which include our credit requirements. Please review our full list of guidelines for more information. By republishing this content, you agree to our republication requirements.
Javascript is disabled
- What's on
- Accessibility
- Travel information
- Food and drink
- Frequently asked questions
- We are changing
- Objects and stories
- The world's first industrial city
- The first railways
- Engineering and mass production
- City of ideas
- Manchester Science Festival
- Researchers
- Press office
- Community partnerships
- Support the museum
- Corporate partnerships
- Gifts in wills
- Trusts, foundations and government
- Volunteering
Science and Industry Museum Liverpool Road Manchester M3 4FP
Please note, the museum will be closed 24–26 December and 1 January. Open daily, book your free museum admission tickets now . Schools and groups can book tickets here . Find out about our ongoing restoration project .
John Dalton: atoms, eyesight and auroras
Published: 16 April 2019
John Dalton (1766–1844) was a Manchester-based scientist whose pioneering work greatly advanced our understanding in multiple fields of research. His surviving apparatus and personal items are now in the Science Museum Group collection.
Who was John Dalton?
Early years and the move to manchester.
Dalton was born in what is now Cumbria in 1766. He became principal at a local Quaker school and taught there until 1793, at which time he moved to Manchester to tutor in natural philosophy and science at the Manchester Academy, a Presbyterian college.
However, his teaching duties left him with too little time to pursue his own scientific interests, so he became a private tutor, including to a budding young scientist called James Prescott Joule (more on whom later).
Joining the 'Lit & Phil'
Soon after moving to Manchester, Dalton joined the Literary & Philosophical Society , which was at the centre of the city's scientific and business community. It was a discussion group set up to share scientific ideas at a time when science had yet to become a profession.
The Society gave him a room for teaching and research at its premises on George Street. Through this, he gained access to a well-equipped research laboratory, where his scientific output flourished.
Though sometimes criticised for the quality of his experiments, Dalton was an enthusiastic investigator who worked late most evenings. He read over 100 papers to the Society, and became its Secretary, Vice-President and, ultimately, President.
Through his experimentation, Dalton not only formulated a new atomic theory to explain chemical reactions, upon which much of modern chemistry and physics is based, but he also developed a theory to explain colour vision deficiency, from which he himself suffered. He was also a figurehead in the world of meteorology.


Atomic theory
Dalton was interested in the composition of the atmosphere and, by extension, in how components mix together to form gases. He formulated the Law of Partial Pressures in 1801, according to which the pressure of a mixed gas is the sum of the pressures that each of its components would exert if occupying the same space. He also developed the law of the thermal expansion of gases.
Henry Roscoe, a later Manchester chemist, suggested that Dalton was trying to explain why the constituents of a gaseous mixture remain homogeneously mixed instead of separating into layers according to their density, the understanding of which is particularly important in atmospheric studies.

At the end of an 1803 paper on the absorption of gases by liquids, Dalton rather casually set out the first table of atomic weights. Encouraged by the favourable reception this paper received, he developed his theory further, in lectures to the Royal Society in 1803–04 and later in his New System of Chemical Philosophy:
Every particle of water is like every other particle of water; every particle of hydrogen is like every other particle of hydrogen... Chemical analysis and synthesis go no farther than to the separation of particles one from another, and to their reunion. No new creation or destruction of matter is within the reach of chemical agency. John Dalton (1808)
Dalton's theory was based on the concept that each element consists of its own unique brand of indivisible atom; atoms of one element are all alike but they differ from atoms of other elements. Importantly, Dalton assigned atomic weights to the atoms of the 20 elements he knew of at the time. This was a revolutionary concept for the day, which would contribute to the development of the periodic table of the elements later in the 19th century.

The below images are reproductions of drawings of atomic formulae by John Dalton, copied from original lent to the Science Museum Group by Manchester Literary and Philosophical Society.

More information about collection object
Why was dalton's work in atomic theory so pioneering.
This concept, that atoms of different elements are distinguished by differences in their weights, opened up new fields of experimentation. Each aspect of Dalton's theory has since been amended or refined, but its overall picture remains as the basis of modern chemistry and physics.
Through his work, Dalton also pioneered the use of ball-and-stick models to illustrate the three-dimensional structure of molecules, which are often used in teaching to this day.
We know when and why Dalton had these models made, because he describes their production and use in a letter written in 1842, two years before his death:
My friend Mr Ewart, at my suggestion, made me a number of equal balls, about an inch in diameter, about 30 years ago; they have been in use ever since, I occasionally show them to my pupils… I had no idea at the time that the atoms were all of a bulk, but for the sake of illustration I had them made alike. From 'Memoirs of the life and scientific researches of John Dalton' by William Charles Henry (1854)
Contemporary critics doubted Dalton's atomic theory and his structural, three-dimensional thinking, as it was far beyond the perceived wisdom of the time. However, his ideas ultimately became fundamental to modern chemistry.
If more chemists had been playing with balls and sticks in the same way as Dalton, the world would not have had to wait so long for the theory of structure. From 'Dalton and Structural Chemistry' by W.V. Farrar (1968)
Colour blindness and 'Daltonism'
In addition to his work with atoms, Dalton also developed a theory to explain colour vision deficiency (or colour blindness), from which he himself suffered. He suggested that the colour of the fluid in the eyes, known as the vitreous humour, acted as a filter to certain colours in the spectrum.
Dalton’s ideas were met with resistance from some of his contemporaries at the time, so to test his theory, Dalton donated his eyes for examination after death. On 28 July 1844, the day after he died, local doctor Joseph Ransome performed the autopsy. 'Perfectly colourless' was the result, proving his theory to be incorrect.
DNA analysis carried out in 1995 and published in the journal Science , 150 years after his death, revealed that Dalton lacked the gene for the receptor sensitive to medium wavelength (green) light, and in fact suffered from deuteranopia, or red-green colour blindness—a condition still referred to as Daltonism.
The eyes were retained by the Literary & Philosophical Society and donated to the museum in 1997.
Watching the weather
In addition to transforming our understanding of chemistry and colour blindness, Dalton was also a fervent weather watcher, becoming an important figure in the field of meteorology. He kept a daily weather diary, producing a detailed record of local weather conditions over 57 years—over 200,000 entries in total. Even in poor health, he continued to journal about the weather, and made his final entry mere hours before his death on 27 July 1844.

As well as the classic Mancunian wind and rain, he also documented sightings of the aurora borealis, becoming enthralled by the 'glowing canopy' of light that occasionally appeared in the skies above the Lake District and Manchester.
You can read more about Dalton's obsession with the weather, particularly his work around the aurora borealis and its causes, on the Science and Industry Museum blog, and more on how space weather affects the Earth over on the website of our sister museum, the Science Museum:

John Dalton and the aurora borealis
The Northern Lights, or aurora borealis, are one of nature's most spectacular phenomena, and have inspired countless artists, explorers, philosophers and scientists over the centuries, including Manchester's own John Dalton.

How does space weather affect the Earth?
In 1859, the largest geomagnetic solar storm on record happened. The impact of this storm, millions of miles away, disrupted the global communications of the day—the telegraph—and showed how the Earth is affected by the activity on the Sun.
What happened to Dalton?
John Dalton was widely honoured in his lifetime. He was elected one of the eight foreign associates of the French Academie des Sciences, a Fellow of the Royal Society and their first Royal Medallist. Oxford and Cambridge Universities both gave him honorary degrees.
Dalton was especially loved by the people of Manchester, so much so that the city paid for a life-size statue to be erected during his lifetime, which can be found in the Town Hall. Upon his death, 40,000 people filed past his coffin as he lay in state, and there were 100 carriages in his funeral procession.
Dalton is now regarded as a rather poor experimenter. However, he had a powerful and vivid pictorial imagination that often gave him profound insights, as exemplified in his work.
Dalton’s scientific connections
Dalton was extremely dedicated to his work and as a result became rather reclusive, remaining unmarried throughout his life and with few friends to speak of. He did, however, have a lasting impact on another 19th-century scientific pioneer: James Joule.

James Prescott Joule (1818–89) is revered as one of the greatest scientists in the history of physics, due to his groundbreaking work in thermodynamics. He was the son of a renowned local brewer and grew up fascinated by all things scientific, and was fortunate enough to be tutored by John Dalton.
Find out more about Joule and his own work here .
Suggestions for further research
- DSL Cardwell (ed.), John Dalton and the progress of science (Manchester: Manchester University Press, 1968)
- F Greenaway, John Dalton and the Atom (Ithaca, NY: Cornell University Press, 1966)
- WC Henry, Memoirs of the life and scientific researches of John Dalton (London: Cavendish Society, 1854)
- RF Hess, LT Sharpe et al. Night Vision: Basic, Clinical and Applied Aspects (Cambridge: Cambridge University Press, 1990)
- AL Smyth (ed.), John Dalton, 1766–1844: a bibliography of works by and about him (Manchester: Manchester Literary and Philosophical Publications, 1998)
- A Thackray, John Dalton: Critical Assessments of His Life and Science (Cambridge, MA: Harvard University Press, 1972)
- Memoirs of the Literary and Philosophical Society of Manchester, Vol. V Part I (Manchester: Manchester Literary and Philosophical Society, 1798)
You may also like

Developing a modern periodic table
The periodic table is one of the most iconic images in science, a guide to the chemistry of our world. But it's only one among many visual ways to classify the elements.

John Dalton in the SMG Collection
Browse all John Dalton-related items in the Science Museum Group collection, from his laboratory equipment and notes to the actual remains of his eyes.

SMG Journal
The Journal presents the global research community with peer-reviewed papers relevant to the work of the Science Museum Group.
- Part of the Science Museum Group
- Terms and conditions
- Privacy and cookies
- Modern Slavery Statement
- Web accessibility
- Skip to content
- Skip to secondary menu
- Skip to primary sidebar
- Skip to footer
Health and Medical Blog
John Dalton’s Atomic Theory Experiment
John Dalton’s atomic theory experiment was the first attempt to describe all matter by way of atoms and their properties in a way that was complete. His theory was based on two verified scientific laws: the law of conservation of mass and the law of constant composition.
The law of conservation of mass says that within a closed system, no matter can be created or destroyed. This means if a chemical reaction happens to create something new, then the amount of each element must come from the same starting materials. It is for this reason that mathematics seeks to create equality and balance.
The law of constant composition says that pure compounds will always have the same proportion of the same elements. That means if you were to look at salt crystals, then you would have the same proportions of the base elements, chlorine and salt, no matter how much salt you had or where you got the salt. Now other items could be added to the salt to change it, but the core atoms of salt are always the same.
The Four Principles of Dalton’s Atomic Theory
When Dalton proposed his atomic theory, it was based on ideas, assumptions, and principles more than facts that were directly observable. This means that there are five components to the atomic theory that are offered by Dalton.
- All matter is made up of atoms. This means that everything that is made of matter is composed of atoms, which are indivisible by design.
- All atoms can be identified by mass and properties. This means that any given element has atoms that must be identical in properties, including their mass. It also means that an element can be identified because its atoms will act like a fingerprint to identify it.
- All compounds are made up of atom combinations. For a compound to form, Dalton suggested with his atomic theory that it would have to be composed of at least two different types of atoms. A combination may also include more than two.
- All chemical reactions are a rearrangement of atoms. This indicates that when a chemical reaction occurs, it is because the atoms are being rearranged in such a way that they form a different combination. It is a whole-number ratio.
- If an element reacts, their atoms may sometimes combine into more than one simple whole-number ratio. This would help to explain why weight ratios in various gases were simple multiples of each other.
Dalton had another postulate that he included with his initial atomic theory that, unfortunately, made it difficult for the scientific community to accept his ideas in their entirety. He believed that when atoms combined in only one ratio, then it needed to be assumed that it would be a binary ratio. This caused him to believe that the formula for water was HO instead of H2O and ammonia was NH instead of NH3.
Dalton had made the same mistake that many had before. Based on his own work, he made an assumption that turned out to not be true. This is why experimentation is so critical to the scientific process.
The Atomic Theory, Experimentations, and Its Modern View
When we look at an atomic theory experiment, what we’re trying to do is either prove that Dalton’s theory is correct or prove that it is incorrect. Evidence must be obtained in order for this to occur, which can only be done through experimentation and observation. Since the theory was first proposed, we have learned quite a lot about atoms and can prove that components of Dalton’s theory are categorically incorrect.
For example: in Principle #1, Dalton stated that atoms were indivisible by design. We know that this is not the case. Atoms are actually made of positive components called protons, negative components called electrons, and neutral components that are called neutrons. Instead of being units that are made up of great mass, atomic theory experiments were able to prove that a vast majority of atoms are basically just empty space.
There are more experiments that have helped to disprove other elements of Dalton’s atomic theory as well, though it would take several generations for scientists to realize that there was a greater truth to find.
The Issue of Neutrons and Isotopes with the Atomic Theory
In Principle #2 of Dalton’s atomic theory, we have found that the idea of atoms having the same mass within a specific element is also incorrect. This is because the number of neutrons that may be present within an atom can vary based on the different isotopes which exist for the same element.
This means Dalton was partially correct, but also partially incorrect. Here’s why.
Let’s take carbon as an example. At the time of this writing, there are 15 known types of carbon that currently exist. Some are natural, while others are artificial. The most stable carbon isotope has a half-life of 5,700 years, while the most stable artificial carbon isotope has a half-life of just 20 minutes. There are actually 3 different occurring isotopes of carbon that occur in nature.
Each isotope is assigned a number. Using the naturally occurring isotopes as an example, they are Carbon-12, Carbon-13, and Carbon-14. These numbers are assigned in such a way not because of the order in which they were discovered, but because each one has a specific isotopic mass.
This means Carbon-8 has an isotopic mass that is close to 8u exactly. Carbon-12 would be 12u. And so forth.
So what the atomic theory experiments regarding atomic number, mass number, and isotopes has been able to determine is this: elements can have different masses. The specific isotopes, however, do not have a different mass. So Dalton was partially correct because you’re not going to find Carbon-14 atoms when you’re looking at Carbon-12. He was partially incorrect because at the time, it was not known that elements could have these different isotope masses.
Dalton’s Atomic Theory and It’s One Missing Item
Maybe you’ve heard of a Quark. No – not the Ferengi bartender on the show Star Trek: Deep Space Nine. Quarks are subatomic particles that carry a fractional electrical charge. They have not been directly observed, but their existence has been predicted and confirmed through experimentation. It is considered to be an elementary particle.
Quarks are considered to be the very building blocks of each atom. They are a primary constituent of neutrons and protons, which means they are part of all ordinary matter. We can determine if an atom will be composing a proton or a neutron because of the number of “up” and “down” quarks that are found.
Two up quarks with one down quark make up a proton. Two down quarks with one up quark make up a neutron.
But these aren’t the only quarks that have been found since Dalton first proposed the atomic theory. Here are some of the other quarks that have been determined to exist.
- Strange Quark. Discovered with the lambda particle, the quark was deemed to be strange because it gave the nucleus of the particle a longer half-life than expected. A lambda particle is a different baryon formation than what creates protons and neutrons. The lambda consists of one up quark, one down quark, and one strange quark.
- Charm Quark. This quark was discovered through experimentation in 1974 and can be transformed into a charm quark.
- Top Quark. Evidence of a third quark was reported in 1995, found through the collision of protons and antiprotons in a collider. Little is known about this quark, other than its mass is quite large compared to other quarks that are believed to exist.
When Dalton was conducting atomic theory experiments, he conducted meteorology experiments because he wanted to prove that evaporated water could exist in the atmosphere as an independent gas. Instead of water molecules and air molecules mixing together, what would happen if it could be proven that they were actually separated?
This caused him to perform experiments on a series of gas mixtures to determine what effect each individual gas may have on the other. Through his observations, he was able to come up with what would become the first version of the atomic theory. It is a process that is still being evaluated to this day.
What Does Dalton’s Atomic Theory Mean Today?
When Dalton first proposed his atomic theory, there was no way to even predict the existence of protons, electrons, and neutrons – much less the existence of quarks or other subatomic particles. Yet when one looks at the entirety of the theory that was offered, many components of it are still considered to be true. It even provides much of the framework that is used in modern chemistry efforts.
Through experimentation, parts of the theory have been modified because of new knowledge. The principles, however, have offered multiple generations of scientists and researchers to know more about the smallest components of our universe. With future experimentation, we can continue to use Dalton’s atomic theory as a foundation for new discoveries.
- 13 ANC Nails Pros and Cons
- 15 Artificial Sphincter Pros and Cons
- 14 Hysterectomy for Fibroids Pros and Cons
- 15 Monovision Lasik Pros and Cons
- 12 Pros and Cons of the Da Vinci Robotic Surgery
- 14 Peritoneal Dialysis Pros and Cons
- 14 Pros and Cons of the Cataract Surgery Multifocal Lens
- 19 Dermaplaning Pros and Cons
- 15 Mirena IUD Pros and Cons
- 11 Pros and Cons of Monovision Cataract Surgery
- Calories Burned
- Cancer Articles and Infographics
- Definitions and Examples of Theory
- Definitions for Kids
- Dental Articles and Infographics
- Elder Care Articles and Infographics
- Environmental
- Health Research Funding
- Healthcare Articles and Infographics
- ICD 9 Codes
- Major Accomplishments
- Medical Articles and Infographics
- Nutrition Articles and Infographics
- Pharmaceutical Articles and Infographics
- Psychological Articles and Infographics
- Skin Articles and Infographics
- Surgery Articles and Infographics
- Theories and Models
- Uncategorized
- Videos on How to Get Research Funding

IMAGES
COMMENTS
During John Dalton's early career, he identified the hereditary nature of red-green color blindness. ... Dalton's experiments on gases led to his discovery that the total pressure of a mixture of ...
Many consider 2008 the 200th anniversary of atomic theory, John Dalton's momentous theory of the nature of matter. Dalton (1766-1844) proposed that all matter in the universe is made of indestructible, unchangeable atoms—each type characterized by a constant mass—that undergo chemical reactions by joining with and separating from each other.
The theory of atomism, proposed by Dalton in the early 19th century and derived from meteorological studies, is the foundation for our modern concept of the atom. ... John Dalton is best known for his pioneering theory of atomism. He also developed methods to calculate atomic weights and structures and formulated the law of partial pressures ...
Science Museum Group Collection More information about Five wooden balls, made by Peter Ewart of Manchester c.1810, and used by John Dalton for demonstrating his atomic theory c. 1810-42 We know when and why Dalton had these models made, because he describes their production and use in a letter written in 1842, two years before his death:
gas method, Dalton also managed somehow to get these results: Parts of oxygen to IOO metal Metal Dalton Modern Silver 7*7 7*4 Mercury 4*0 4-o Copper 125 I2-6 Nickel 27 *O 27.3 In Dalton's work on the neutral salts we see plainly the influence of Richter on the development of his chemical atomic theory.2- Dalton
John Dalton (born September 5 or 6, 1766, Eaglesfield, Cumberland, England—died July 27, 1844, Manchester) was an English meteorologist and chemist, a pioneer in the development of modern atomic theory.. Early life and education. Dalton was born into a Quaker family of tradesmen; his grandfather Jonathan Dalton was a shoemaker, and his father, Joseph, was a weaver.
John Dalton, the father of modern atomic theory. Credit: chemheritage.org In 1803, Dalton orally presented his first list of relative atomic weights for a number of substances.
John Dalton - Atomic Theory, Chemistry, Physics: By far Dalton's most influential work in chemistry was his atomic theory. Attempts to trace precisely how Dalton developed this theory have proved futile; even Dalton's own recollections on the subject are incomplete. He based his theory of partial pressures on the idea that only like atoms in a mixture of gases repel one another, whereas ...
John Dalton's atomic theory experiment was the first attempt to describe all matter by way of atoms and their properties in a way that was complete. His theory was based on two verified scientific laws: the law of conservation of mass and the law of constant composition. The law of conservation of mass says that …
Dalton′s discovery: The experiments that led to Dalton's discovery of multiple combining proportions are judged by some to be irreproducible. A successful replication and analysis exonerates Dalton but suggests atomistic preconceptions shaped his experimental protocol to deliver the desired result.