
- Why Does Water Expand When It Freezes
Gold Foil Experiment
- Faraday Cage
- Oil Drop Experiment
- Magnetic Monopole
- Why Do Fireflies Light Up
- Types of Blood Cells With Their Structure, and Functions
- The Main Parts of a Plant With Their Functions
- Parts of a Flower With Their Structure and Functions
- Parts of a Leaf With Their Structure and Functions
- Why Does Ice Float on Water
- Why Does Oil Float on Water
- How Do Clouds Form
- What Causes Lightning
- How are Diamonds Made
- Types of Meteorites
- Types of Volcanoes
- Types of Rocks

Who did the Gold Foil Experiment?
The gold foil experiment was a pathbreaking work conducted by scientists Hans Geiger and Ernest Marsden under the supervision of Nobel laureate physicist Ernest Rutherford that led to the discovery of the proper structure of an atom . Known as the Geiger-Marsden experiment, it was performed at the Physical Laboratories of the University of Manchester between 1908 and 1913.
The prevalent atomic theory at the time of the research was the plum pudding model that was developed by Lord Kelvin and further improved by J.J. Thomson. According to the theory, an atom was a positively charged sphere with the electrons embedded in it like plums in a Christmas pudding.
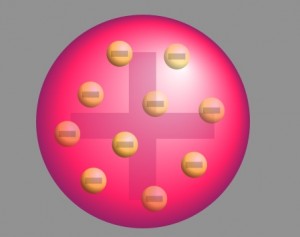
With neutrons and protons yet to be discovered, the theory was derived following the classical Newtonian Physics. However, in the absence of experimental proof, this approach lacked proper acceptance by the scientific community.
What is the Gold Foil Experiment?
Description.
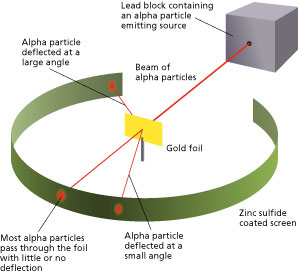
The method used by scientists included the following experimental steps and procedure. They bombarded a thin gold foil of thickness approximately 8.6 x 10 -6 cm with a beam of alpha particles in a vacuum. Alpha particles are positively charged particles with a mass of about four times that of a hydrogen atom and are found in radioactive natural substances. They used gold since it is highly malleable, producing sheets that can be only a few atoms thick, thereby ensuring smooth passage of the alpha particles. A circular screen coated with zinc sulfide surrounded the foil. Since the positively charged alpha particles possess mass and move very fast, it was hypothesized that they would penetrate the thin gold foil and land themselves on the screen, producing fluorescence in the part they struck.
Like the plum pudding model, since the positive charge of atoms was evenly distributed and too small as compared to that of the alpha particles, the deflection of the particulate matter was predicted to be less than a small fraction of a degree.
Observation
Though most of the alpha particles behaved as expected, there was a noticeable fraction of particles that got scattered by angles greater than 90 degrees. There were about 1 in every 2000 particles that got scattered by a full 180 degree, i.e., they retraced their path after hitting the gold foil.
Simulation of Rutherford’s Gold Foil Experiment Courtesy: University of Colorado Boulder
The unexpected outcome could have only one explanation – a highly concentrated positive charge at the center of an atom that caused an electrostatic repulsion of the particles strong enough to bounce them back to their source. The particles that got deflected by huge angles passed close to the said concentrated mass. Most of the particles moved undeviated as there was no obstruction to their path, proving that the majority of an atom is empty.
In addition to the above, Rutherford concluded that since the central core could deflect the dense alpha particles, it shows that almost the entire mass of the atom is concentrated there. Rutherford named it the “nucleus” after experimenting with various gases. He also used materials other than gold for the foil, though the gold foil version gained the most popularity.
He further went on to reject the plum pudding model and developed a new atomic structure called the planetary model. In this model, a vastly empty atom holds a tiny nucleus at the center surrounded by a cloud of electrons. As a result of his gold foil experiment, Rutherford’s atomic theory holds good even today.
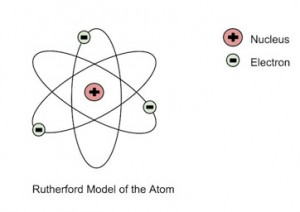
Rutherford’s Atomic Model
Rutherford’s Gold Foil Experiment Animation
- Rutherford demonstrated his experiment on bombarding thin gold foil with alpha particles contributed immensely to the atomic theory by proposing his nuclear atomic model.
- The nuclear model of the atom consists of a small and dense positively charged interior surrounded by a cloud of electrons.
- The significance and purpose of the gold foil experiment are still prevalent today. The discovery of the nucleus paved the way for further research, unraveling a list of unknown fundamental particles.
- Chemed.chem.purdue.edu
- Chem.libretexts.org
- Large.stanford.edu
- Radioa ctivity.eu.com
Article was last reviewed on Friday, February 3, 2023
Related articles
5 responses to “Gold Foil Experiment”
Super very much helpful to me,clear explanation about every act done by our Rutherford that is under different sub headings ,which is very much clear to ,to study .very much thanks to the science facts.com.thank u so much.
Good explanation,very helpful ,thank u ,so much
very clear and helpful, perfect for my science project!
Thank you for sharing the interactive program on the effects of the type of atom on the experiment! Looking forward to sharing this with my ninth graders!
Rutherford spearheaded with a team of scientist in his experiment of gold foil to capture the particles of the year 1911. It’s the beginning of explaining particles that float and are compacted . Rutherford discovered this atom through countless experiments which was the revolutionary discovery of the atomic nuclear . Rutherford name the atom as a positive charge and the the center is the nucleus.
Barack Hussein Obama
Mrs. Danize Obama
Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.
Popular Articles

Join our Newsletter
Fill your E-mail Address

Related Worksheets
- Privacy Policy
© 2024 ( Science Facts ). All rights reserved. Reproduction in whole or in part without permission is prohibited.
Ernest Rutherford

(1871-1937)
Who Was Ernest Rutherford?
A pioneer of nuclear physics and the first to split the atom, Ernest Rutherford was awarded the 1908 Nobel Prize in Chemistry for his theory of atomic structure. Dubbed the “Father of the Nuclear Age,” Rutherford died in Cambridge, England, on October 19, 1937, of a strangulated hernia.
Early Life and Education
Ernest Rutherford was born in rural Spring Grove, on the South Island of New Zealand on August 30, 1871. He was the fourth of 12 children and the second son. His father, James, had little education and struggled to support the large family on a flax millers' income. Ernest’s mother, Martha, worked as a schoolteacher. She believed that knowledge was power, and placed a strong emphasis on her children’s education.
As a child, Ernest, whose family called him “Ern,” spent most of his time after school milking cows and helping with other chores on the family farm. Weekends were spent swimming in the creek with his brothers. Since money was tight, Rutherford found inventive ways of overcoming his family’s financial challenges, including birds-nesting to earn funds for his kite-flying supplies. “We haven’t the money, so we’ve got to think,” was Rutherford’s motto at the time.
At the age of 10, Rutherford was handed his first science book, at Foxhill School. It was a pivotal moment for Rutherford, given that the book inspired his very first scientific experiment. The young Rutherford constructed a miniature cannon, which, to his family’s surprise, promptly and unexpectedly exploded. Despite the outcome, Rutherford’s interest in academics remained unfaltering. In 1887 he was awarded a scholarship to attend Nelson Collegiate School, a private secondary school where he would board and play rugby until 1889.
In 1890 Rutherford landed another scholarship—this time to Canterbury College in Christchurch, New Zealand. At Canterbury College, Rutherford’s professors fueled his enthusiasm for seeking concrete proof through scientific experimentation. Rutherford obtained both his Bachelor of Arts and his Master of Arts degrees there, and managed to achieve first-class honors in math and science. In 1894, still at Canterbury, Rutherford conducted independent research on the ability of high-frequency electrical discharge to magnetize iron. His research earned him a Bachelor of Science degree in just one year’s time. During that same year, Rutherford met and fell in love with his landlady’s daughter, Mary Newton. The couple married in 1900 and later welcomed a daughter, whom they named Eileen.
Experiments and Discoveries
In 1895, as the first research student at the University of Cambridge’s Cavendish Laboratory in London, Rutherford identified a simpler and more commercially viable means of detecting radio waves than had been previously established by German physicist Heinrich Hertz.
Also while at Cavendish Laboratory, Rutherford was invited by Professor J.J. Thomson to collaborate on a study of X-rays. German physicist Wilhelm Conrad Röntgen had discovered X-rays just months before Rutherford arrived at Cavendish, and X-rays were a hot topic among research scientists. Together, Rutherford and Thomson studied the effects of X-rays on the conductivity of gases, resulting in a paper about dividing atoms and molecules into ions. While Thomson went on to examine what would later be called an electron, Rutherford took a closer look at ion-producing radiations.
Focusing on uranium, Rutherford discovered that placing it near foil resulted in one type of radiation being easily soaked up or blocked, while a different type had no trouble penetrating the same foil. He labeled the two radiation types “alpha” and “beta.” As it turns out, the alpha particle was identical to the nucleus of a helium atom. The beta particle was, in fact, the same as an electron or positron.
Rutherford left Cambridge in 1902 and took up a professorship at McGill University in Montreal. At McGill in 1903, Rutherford and has colleague Frederick Soddy introduced their disintegration theory of radioactivity, which claimed radioactive energy was emitted from within an atom and that when alpha and beta particles were emitted at the same time, they caused a chemical change across elements. Rutherford and Yale Professor Bertram Borden Boltwood went on to categorize radioactive elements into what they called a “decay series.” Rutherford was also credited with discovering the radioactive gas radon while at McGill. Achieving fame for his contributions to the understanding of radioelements, Rutherford became an active public speaker, published numerous magazine articles and wrote the most highly regarded textbook of the time on radioactivity.
In 1907, Rutherford returned to England, transferring to a professorship at the University of Manchester. Through further experimentation involving firing alpha particles at foil, Rutherford made the groundbreaking discovery that nearly the total mass of an atom is concentrated in a nucleus. In so doing, he gave birth to the nuclear model, a discovery that marked the inception of nuclear physics and ultimately paved the way to the invention of the atom bomb. Aptly dubbed the “Father of the Nuclear Age,” Rutherford received the Nobel Prize for Chemistry in 1908.
With the advent of World War I, Rutherford turned his attention to antisubmarine research. By 1919 he had made another monumental discovery: how to artificially induce a nuclear reaction in a stable element. Nuclear reactions were Rutherford’s main focus for the rest of his scientific career.
Death and Legacy
Rutherford was awarded countless honors during his career, including several honorary degrees and fellowships from organizations such as the Institution of Electrical Engineers. In 1914 he was knighted. In 1931, he was elevated to the peerage, and granted the title Baron Rutherford of Nelson. He was also elected president of the Institute of Physics that same year.
On October 19, 1937, Baron Rutherford died in Cambridge, England at age 66 from the complications of a strangulated hernia. The scientist, who had been nicknamed “Crocodile” by his colleagues for always looking ahead, was buried at Westminster Abbey.
Years before he died, during World War I, Rutherford said he hoped scientists would not learn how to extract atomic energy until “man was living at peace with his neighbors.” The discovery of nuclear fission was, in fact, made just two years after his death, and eventually resulted in what Rutherford had feared—the use of nuclear power to build wartime weapons.
Many of Rutherford’s discoveries also became the basis of the European Organization for Nuclear Research’s construction of the Large Hadron Collider. The largest and highest-energy particle accelerator in the world and decades in the making, the Large Hadron Collider started smashing atomic particles in May 2010. It has since been used to answer fundamental questions about physics, by scientists who share in Rutherford’s tendency toward forward-thinking and his relentless quest for proof through scientific exploration.
QUICK FACTS
- Name: Ernest Rutherford
- Birth Year: 1871
- Birth date: August 30, 1871
- Birth City: Spring Grove
- Birth Country: New Zealand
- Gender: Male
- Best Known For: Physicist Ernest Rutherford was the central figure in the study of radioactivity who led the exploration of nuclear physics.
- Science and Medicine
- Astrological Sign: Virgo
- Canterbury College
- Nelson Collegiate School
- Nacionalities
- New Zealander
- Death Year: 1937
- Death date: October 19, 1937
- Death City: Cambridge
- Death Country: United Kingdom
We strive for accuracy and fairness.If you see something that doesn't look right, contact us !
CITATION INFORMATION
- Article Title: Ernest Rutherford Biography
- Author: Biography.com Editors
- Website Name: The Biography.com website
- Url: https://www.biography.com/scientists/ernest-rutherford
- Access Date:
- Publisher: A&E; Television Networks
- Last Updated: November 5, 2021
- Original Published Date: April 2, 2014

Famous British People

Ralph Fiennes

Liam Payne’s Girlfriend Speaks Out After His Death

Daniel Day-Lewis

Maggie Smith

Alan Cumming

Olivia Colman

Richard III

20 Shakespeare Quotes

William Shakespeare
Exploring Radioactivity
Rutherford at mcgill university, 1898–1907.
J.J. Thomson wrote supporting Rutherford for the MacDonald Chair at McGill University:
“I have never had a student with more enthusiasm or ability for original research than Mr Rutherford and I am sure that if elected he would establish a distinguished school of Physics at Montreal.”

Good history of science is written as if looking over the shoulders of researchers as they worked before they had figured out their answers. When Ernest Rutherford accepted the MacDonald Professorship at McGill University in Montreal, Canada, in 1898, little was known about radioactivity or radiation. As he wrote of part of this puzzle: “The cause and origin of the radiations continuously emitted by uranium still remain a mystery.” (Rutherford, 1962, 1:214). Rutherford saw a mystery.
Becquerel had discovered in 1896 that uranium gives off invisible rays that fog photographic film. The Curies had shown that thorium acts similarly. In November 1898, they reported the chemical separation of two unknown materials from the ore pitchblende, both highly radioactive — the first use of the word. André Debierne (1874–1949) discovered another radioactive element, actinium. What they had was unclear, but it was spectacularly unexpected.
The challenge these unexpected phenomena presented had been faced by scientists before. How does one explain something completely new? Isaac Newton (1642–1727) had faced this with light: what is color, what produces fringes of light and shadow at the edges of objects? Michael Faraday (1791–1867) discovered that changes in magnetism produce electrical current and that electromagnetic forces may bend and flex. They investigated these new phenomena by exhaustive experimentation and description. Rutherford's work on radioactivity bears a strong resemblance.

The method to this experimentation is simple: joyful and inventive play. Think of every tool or technique that might tell us something about the rays given off by radioactive materials. Try them all out. Many will show no result, but some will be revealing. Becquerel found that his rays could pass through thick, opaque materials and that they could ionize gasses so that electrical currents could pass through. He also thought that his rays can be refracted and polarized, similarly to light. Rutherford, however, repeated and perfected these experiments. He detected no refraction or polarization.
Just before he left Cambridge for Montreal in 1898, Rutherford conducted a simple, systematic experiment to study the absorption of rays from uranium. Between a uranium source and an electroscope detector, he placed first one thickness of aluminum foil, then two, and so on up to 13. In each stage, he measured the intensity of the radiation by measuring the time required to discharge the electroscope. He found “at least two distinct types of radiation” which he called α (alpha) and β (beta), “for convenience.” He tested different metallic foils, different radioactive sources, etc. Later, he used other means to manipulate these α and β rays and determined that they were, respectively, positively charged and negatively charged particles. In 1901 he determined that Becquerel's rays are indeed electromagnetic rays. He called them γ (gamma) rays.

The second important characteristic of Rutherford's research at McGill University was his balancing of easy hypothesizing with an absolute reluctance to commit to a conclusion until experimental evidence was indisputable. The α particle provides the best example. Rutherford very carefully established the identity and properties of the α particle between 1898 and 1907. His attempts to deflect α particles with electric fields failed at first, but greater attention to the apparatus led to success. He could show that it was positively charged and he suspected that these particles were doubly ionized helium, that is, the helium nucleus. He refrained from saying this, however, because the challenge of measuring that charge proved insurmountable while he was at McGill. As he wrote to his friend Otto Hahn on 6 January 1907:

“In regard to the alpha particle and helium, etc., and the fitting of atomic weights, I am not worrying about it at present. The whole problem is very mixed. The difficulty lies in the fact that ‘the alpha particle moves in a peculiar way its wonders to perform.’ ...We want a new method of attack.” (Quoted in Eve, 1939, pp. 153–4)
He found that new method in Manchester in 1908, while working with his student Thomas Royds (1884–1955).

As a result, Rutherford's best known results from his years at McGill University were those contained in his texts Radio-activity (1st ed. 1904, 2nd ed. 1905) and Radioactive Transformations (1906). These secured his reputation and ultimately a Nobel Prize.
Rutherford left McGill University in 1907. Why did he leave good facilities and colleagues? He wrote in that same letter to Hahn:
“I shall be glad to be nearer the scientific centre as I always feel America as well as Canada is on the periphery of the circle.”
Home | Sections | Credits | Exhibit Hall | About Us

IMAGES
VIDEO
COMMENTS
Oct 23, 2024 · Ernest Rutherford, British physicist who discovered that the atom is mostly empty space surrounding a massive nucleus and who did many pioneering experiments with radioactivity. He was also known for predicting the existence of the neutron and calculating Avogadro’s number. He won the Nobel Prize for Chemistry in 1908.
Oct 23, 2024 · Rutherford model, description of the structure of atoms proposed (1911) by the New Zealand-born physicist Ernest Rutherford. The model described the atom as a tiny, dense, positively charged core called a nucleus, around which the light, negative constituents, called electrons, circulate at some distance.
Ernest Rutherford, 1st Baron Rutherford of Nelson, (30 August 1871 – 19 October 1937), was a New Zealand physicist who was a pioneering researcher in both atomic and nuclear physics. He has been described as "the father of nuclear physics", [ 7 ] and "the greatest experimentalist since Michael Faraday ". [ 8 ]
Explore the nuclear model of the atom and Rutherford's experiment that led to its discovery on this webpage.
Feb 3, 2023 · The gold foil experiment was a pathbreaking work conducted by scientists Hans Geiger and Ernest Marsden under the supervision of Nobel laureate physicist Ernest Rutherford that led to the discovery of the proper structure of an atom. Known as the Geiger-Marsden experiment, it was performed at the Physical Laboratories of the University of ...
In the now well-known experiment, alpha particles were observed to scatter backwards from a gold foil. Rutherford’s explanation, which he published in May 1911, was that the scattering was caused by a hard, dense core at the center of the atom–the nucleus. Ernest Rutherford was born in New Zealand, in 1871, one of 12 children.
Apr 2, 2014 · (1871-1937) Who Was Ernest Rutherford? A pioneer of nuclear physics and the first to split the atom, Ernest Rutherford was awarded the 1908 Nobel Prize in Chemistry for his theory of atomic structure.
Rutherford tried to reconcile scattering results with different atomic models, especially that of J.J. Thomson, in which the positive electricity was considered as dispersed evenly throughout the whole sphere of the atom. A page of Rutherford's early, undated (1910 or 1911), rough notes. The first few lines read: "Theory of structure of atom.
Oct 8, 2024 · Rutherford overturned Thomson’s model in 1911 with his famous gold-foil experiment, in which he demonstrated that the atom has a tiny, massive nucleus. Five years earlier Rutherford had noticed that alpha particles beamed through a hole onto a photographic plate would make a sharp-edged picture, while alpha particles beamed through a sheet of mica only 20 micrometres (or about 0.002 cm ...
When Ernest Rutherford accepted the MacDonald Professorship at McGill University in Montreal, Canada, in 1898, little was known about radioactivity or radiation. As he wrote of part of this puzzle: “The cause and origin of the radiations continuously emitted by uranium still remain a mystery.” (Rutherford, 1962, 1:214). Rutherford saw a ...